
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
Original Articles
- Drug Regimen
- Efficacy and Safety of Enavogliflozin versus Dapagliflozin as Add-on to Metformin in Patients with Type 2 Diabetes Mellitus: A 24-Week, Double-Blind, Randomized Trial
- Kyung Ah Han, Yong Hyun Kim, Doo Man Kim, Byung Wan Lee, Suk Chon, Tae Seo Sohn, In Kyung Jeong, Eun-Gyoung Hong, Jang Won Son, Jae Jin Nah, Hwa Rang Song, Seong In Cho, Seung-Ah Cho, Kun Ho Yoon
- Diabetes Metab J. 2023;47(6):796-807. Published online February 9, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0315
- 40,296 View
- 583 Download
- 5 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
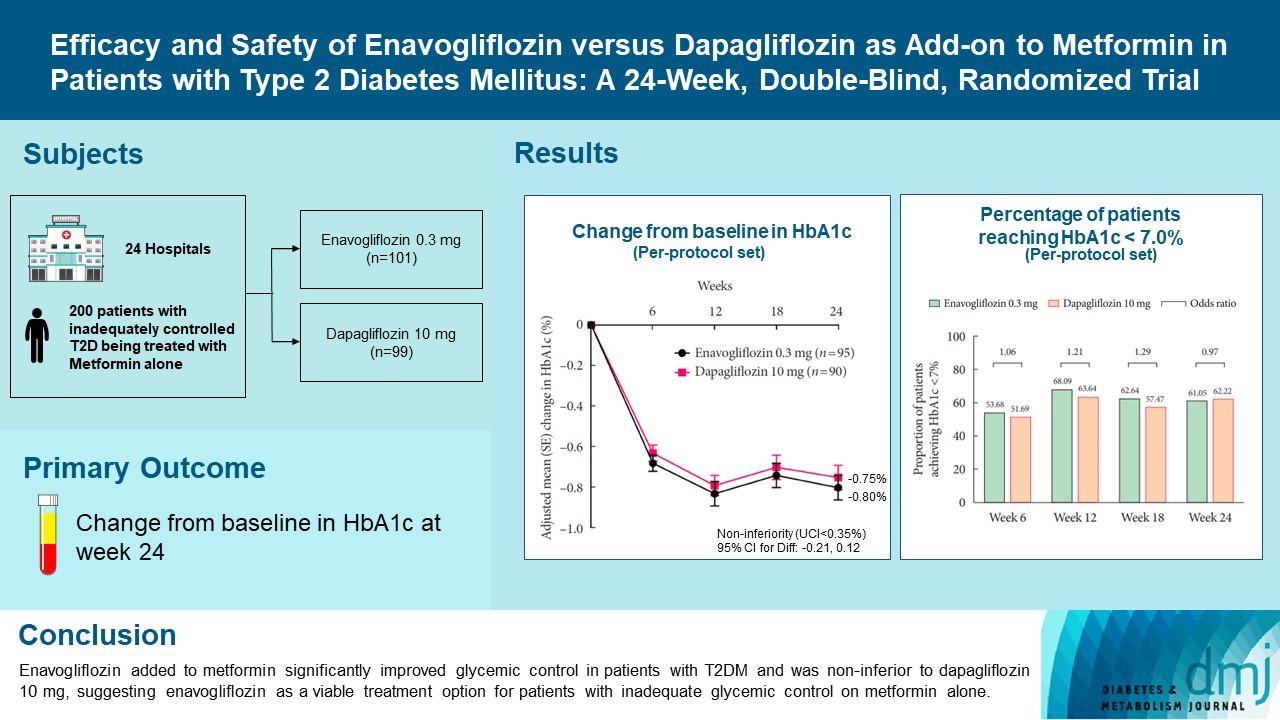
Enavogliflozin is a novel sodium-glucose cotransporter-2 inhibitor currently under clinical development. This study evaluated the efficacy and safety of enavogliflozin as an add-on to metformin in Korean patients with type 2 diabetes mellitus (T2DM) against dapagliflozin.
Methods
In this multicenter, double-blind, randomized, phase 3 study, 200 patients were randomized to receive enavogliflozin 0.3 mg/day (n=101) or dapagliflozin 10 mg/day (n=99) in addition to ongoing metformin therapy for 24 weeks. The primary objective of the study was to prove the non-inferiority of enavogliflozin to dapagliflozin in glycosylated hemoglobin (HbA1c) change at week 24 (non-inferiority margin of 0.35%) (Clinical trial registration number: NCT04634500).
Results
Adjusted mean change of HbA1c at week 24 was –0.80% with enavogliflozin and –0.75% with dapagliflozin (difference, –0.04%; 95% confidence interval, –0.21% to 0.12%). Percentages of patients achieving HbA1c <7.0% were 61% and 62%, respectively. Adjusted mean change of fasting plasma glucose at week 24 was –32.53 and –29.14 mg/dL. An increase in urine glucose-creatinine ratio (60.48 vs. 44.94, P<0.0001) and decrease in homeostasis model assessment of insulin resistance (–1.85 vs. –1.31, P=0.0041) were significantly greater with enavogliflozin than dapagliflozin at week 24. Beneficial effects of enavogliflozin on body weight (–3.77 kg vs. –3.58 kg) and blood pressure (systolic/diastolic, –5.93/–5.41 mm Hg vs. –6.57/–4.26 mm Hg) were comparable with those of dapagliflozin, and both drugs were safe and well-tolerated.
Conclusion
Enavogliflozin added to metformin significantly improved glycemic control in patients with T2DM and was non-inferior to dapagliflozin 10 mg, suggesting enavogliflozin as a viable treatment option for patients with inadequate glycemic control on metformin alone. -
Citations
Citations to this article as recorded by- Efficacy and safety of enavogliflozin vs. dapagliflozin as add-on therapy in patients with type 2 diabetes mellitus based on renal function: a pooled analysis of two randomized controlled trials
Young Sang Lyu, Sangmo Hong, Si Eun Lee, Bo Young Cho, Cheol-Young Park
Cardiovascular Diabetology.2024;[Epub] CrossRef - A 52‐week efficacy and safety study of enavogliflozin versus dapagliflozin as an add‐on to metformin in patients with type 2 diabetes mellitus: ENHANCE‐M extension study
Tae Seo Sohn, Kyung‐Ah Han, Yonghyun Kim, Byung‐Wan Lee, Suk Chon, In‐Kyung Jeong, Eun‐Gyoung Hong, Jang Won Son, JaeJin Na, Jae Min Cho, Seong In Cho, Wan Huh, Kun‐Ho Yoon
Diabetes, Obesity and Metabolism.2024; 26(6): 2248. CrossRef - The effect of renal function on the pharmacokinetics and pharmacodynamics of enavogliflozin, a potent and selective sodium‐glucose cotransporter‐2 inhibitor, in type 2 diabetes
Sae Im Jeong, Mu Seong Ban, Jun‐Gi Hwang, Min‐Kyu Park, Soo Lim, Sejoong Kim, Soon Kil Kwon, Yoonjin Kim, Jae Min Cho, Jae Jin Na, Wan Huh, Jae‐Yong Chung
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - Role of novel sodium glucose co-transporter-2 inhibitor enavogliflozin in type-2 diabetes: A systematic review and meta-analysis
Deep Dutta, B.G. Harish, Beatrice Anne, Lakshmi Nagendra
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(8): 102816. CrossRef - Characteristics of the Latest Therapeutic Agent for Diabetes
Nuri Yun
The Journal of Korean Diabetes.2023; 24(3): 148. CrossRef - Prospects of using sodium-glucose co-transporter-2 (SGLT-2) inhibitors in patients with metabolic-associated fatty liver disease (MAFLD)
Iryna Kostitska, Nadia Protas, Liliia Petrovska
Diabetes Obesity Metabolic Syndrome.2023; (5): 8. CrossRef - Navigating the Future of Diabetes Treatment with New Drugs: Focusing on the Possibilities and Prospects of Enavogliflozin
Sang Youl Rhee
Diabetes & Metabolism Journal.2023; 47(6): 769. CrossRef
- Efficacy and safety of enavogliflozin vs. dapagliflozin as add-on therapy in patients with type 2 diabetes mellitus based on renal function: a pooled analysis of two randomized controlled trials
- Effect of Valsartan on Blood Pressure and Urinary Albumin Excretion in Hypertensive Type 2 Diabetic Patients: An Open-Label, Multicenter Study.
- Se Jun Park, Dae Jung Kim, Hae Jin Kim, Soo Yeon Park, Ji A Seo, Nan Hee Kim, Sung Hee Choi, Soo Lim, Hak Chul Jang, Seung Hyun Ko, Ki Ho Song, Yu Bae Ahn, Soo Kyoung Kim, Yong Wook Cho, Jun Goo Kang, Sung Hee Ihm, Cheol Young Park, Sung Woo Park, Dong Hyun Shin, Yong Hyun Kim, Kwan Woo Lee
- Korean Diabetes J. 2008;32(6):513-521. Published online December 1, 2008
- DOI: https://doi.org/10.4093/kdj.2008.32.6.513
- 2,319 View
- 25 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Activation of renin-angiotensin system (RAS) has been an important mechanism of microvascular and macrovascular complications in diabetic patients. It has been reported that RAS blockades reduce the development and progression of diabetic nephropathy. The aim of this study was to evaluate whether valsartan, an angiotensin II receptor blocker (ARB), reduced blood pressure and urinary albumin excretion rate (UAER) in hypertensive type 2 diabetic patients. METHOD: Three hundred forty-seven hypertensive type 2 diabetic patients who had not taken angiotensin converting enzyme inhibitors or ARB for 6 months prior to this study were enrolled. We measured blood pressure and UAER before and after 24 weeks of valsartan treatment. RESULT: Baseline mean systolic and diastolic blood pressure was 143 +/- 15 and 87 +/- 11 mmHg, respectively and the median albumin excretion rate was 27 ug/mg. Reduction in systolic and diastolic blood pressure was 16 mmHg/10 mmHg and the median UAER was 19.3 ug/mg after 24 weeks (P < 0.01, respectively). When we divided the subjects into three groups according to the UAER (normoalbuminuria, microalbuminuria and macroalbuminuria), significant changes were reported in the microalbuminuria and the macroalbuminuria groups. Thirty-eight (42%) patients with microalbuminuria improved to normoalbuminuria and twelve (41%) patients with macroalbuminuria improved to microalbuminuria. We found an association between the improvement of blood pressure and UAER (R = 0.165, P = 0.015). CONCLUSION: We concluded that valsartan reduces urinary albumin excretion and blood pressure in hypertensive type 2 diabetic patients.
- Polymorphism of the Uncoupling Protein 1 (UCP-1) Gene and Fatty Acid Binding Protein 2 (FABP2) Gene in Korean Type 2 Diabetic Patients.
- Sun Gyu Kim, Chul Hee Kim, Seog Ki Yun, Yeo Il Yun, Yong Hyun Kim, Il Song Nam, Ju Young Lee, Ji O Mok, Hyeong Kyu Park, Young Sun Kim, Dong Won Byun, Kyo Il Suh, Myung Hi Yoo
- Korean Diabetes J. 2001;25(4):262-272. Published online August 1, 2001
- 1,124 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
It is well known that genetic component plays an important role in developing obesity and type 2 diabetes mellitus. A number of candidate genes have been suggested, but the major gene determining the development of obesity and type 2 diabetes has not yet been uncovered. Previous studies suggest that polymorphisms of the intestinal fatty acid binding protein (FABP2) and uncoupling protein 1 (UCP-1) gene were related with obesity and/or insulin resistance in several populations. METHODS: We examined 76 type 2 diabetic patients (aged 44+/-6 years) and 96 healthy controls (aged 25+/-3 years). Ala54Thr polymorphism of the FABP2 gene and A to G polymorphism (-3826) of the UCP-1 gene were determined by polymerase chain reaction and restriction fragment length polymorphism method. RESULTS: The Thr54 allele of the FABP2 gene was found with a frequency of 0.33 in nondiabetic controls and 0.36 in type 2 diabetic patients. The genotype frequency of the Ala54Thr polymorphism was similar in nondiabetic and diabetic subjects ( 2=0.87, P=0.64). The -3826 G allele of UCP-1 gene was found with a frequency of 0.51 in nondiabetic controls, and 0.46 in type 2 diabetic patients. The genotype frequency of the -3826 A to G polymorphism was also similar in nondiabetic and diabetic subjects ( 2=1.46, p=0.46). When the subjects of each groups were subdivided into nonobese and obese group by BMI of 25 kg/m2, there was no significant difference in genotype frequencies of the UCP-1 and FABP2 gene polymorphisms. CONCLUSION: These results suggest that either the Ala54Thr polymorphism of the FABP2 gene or the A to G polymorphism (-3826) of UCP-1 gene do not play a major role in developing type 2 diabetes mellitus or obesity in Korean.
- The Relation Between DHEA, DHEAS and Syndrome X, Cardiovascular Complication in Type 2 Diabetes Mellitus.
- Yong Hyun Kim, Jeong Heon Oh, Nan Hee Kim, Kyung Mook Choi, Sang Jin Kim, Sei Hyun Baik, Dong Seop Choi
- Korean Diabetes J. 2000;24(2):234-244. Published online January 1, 2001
- 1,057 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Insulin is known as a major factor that regulates secretion of DHEA and DHEAS. Numerous studies are exist to investigate the relationship between DHEA(S) and insulin resistance. Furthermore, numerous previous studies revealed that insulin resistance plays a major role in the pathogenic relationship between DHEA(S) and type 2 diabetes mellitus. However, number of studies to investigate the difference of levels of DHEA(S) according to the presence of syndrome X in type 2 diabetes mellitus are limited. METHODS: In type 2 diabetes, aged from 40 to 70 years old, the levels of serum DHEA and DHEAS was compared between the subejcts with or without syndrome X as well as the normal age and sex matched control. Furthermore, correlation between serum DHEA/DHEAS and insulin resistance, and the levels of DHEA/DHEAS according to the cardiovascular complication status was also evaluated. RESULTS: 1. No statistical difference in serum DHEA and DHEAS was observed among the 3 groups. However, the serum DHEA and DHEAS levels were lower in type 2 diabetes with syndrome X and higher in normal control. 2. No correlation was observed between DHEA, DHEAS and insulin resistance factors. 3. No stastistical difference in serum DHEA and DHEAS was observed in type 2 diabetic patients with cardiovascular complications. However, the level of DHEA was lower in the patients with cardiovascular complications. 4. No stastistical difference in serum DHEA and DHEAS was observed according to the presence of cardiovascular complications when analysis was performed in 55 years and younger subjects. 5. The level of DHEA was lower in the presence of cardiovascular complication when only male diabetic subjects were included in the analysis, but the level of DHEAS showed no difference according to the cardiovascular complication status. CONCLUSION: No statistical difference of the levels of serum OHEA and DHEAS was observed according to the presence of syndrome X in type 2 diabetes patients, However, the level of serum DHEA tended to be lower in the presence of cardiovascular complications. The levels of DHEA in male diabetic subjects were lower in the presence of cardiovascular complication, thus, we suspected that DHEA may play a potential role as one of risk factors of cardiovascular complications in this subgroup.
- Effects of Insulin and Vitamin E on the Apoptosis of Pancreatic Islet Cells in Multiple Low dose Streptozotocin Induced Diabetic (LDSD) Mice.
- Yong Hyun Kim, Jeong Hun Oh, Nan Hee Kim, Kyung Muk Choi, Sang Jin Kim, Sei Hyun Baik, Eung Seok Lee, Min Chul Lee, Dong Seop Choi
- Korean Diabetes J. 1999;23(6):757-767. Published online January 1, 2001
- 1,028 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Type 1 diabetes mellitus results from irreversible loss of beta cells in pancreatic islet. It is generally known that abnormal MHC expression and interaction of variable cytokines play a role in beta cell death, but the precise mechanism of beta cell death is unknown. Apoptosis is a physiological form of cell death and can play an important role in beta cell death in experimental diabetic animal models. Thus, in insulin and vitamin E treated LDSD mice and streptozotocin treated control mice. We attempted to comparing the levels of blood glucose (BG), the degree of insulitis, and number of apoptotic cells. Our study goal was to understand inhibition of apoptosis which thought to play an important mechanism in reducing the degree of hyperglycemia and insulitis. METHODS: In 3 LDSD mice groups (group 1: control group with streptozotocin only, group 2: streptozotocin plus insulin, group 3: streptozotocin plus vitamin E), the effects of insulin and vitamin E on the blood glucose levels and the degree of insulitis were evaluated. The number of apoptotic cells of pancreatic islet was compared using double staining immunohistochemical method. RESULT: The levels of BG, degree of insulitis and the rate of apoptosis of pancreatic islet cells were decreased in insulin and vitamin E treated groups when compared to the control group. There was no difference in number of apoptotic cells between insulin and vitamin E treated group, but levels of BG and degree of insulitis were higher in vitamin E treated group than insulin treated group as time elapsed. CONCLUSION: Insulin and vitamin E can decrease the elevation of BG and the degree of insulitis via inhibition of apoptosis in LDSD mice.
Case Report
- A Case of Severe Hypertriglyceridemia with Diabetic Ketoacidosis.
- Dong Seop Choi, Jeong Heon Oh, Ie Byung Park, Jin Won Kim, Kyung Mook Choi, Yong Hyun Kim, Nan Hee Kim, Sang Jin Kim, Sei Hyun Baik
- Korean Diabetes J. 1999;23(5):715-721. Published online January 1, 2001
- 1,066 View
- 21 Download
-
 Abstract
Abstract
 PDF
PDF - Severe hypertriglyceridemia exceeding 5.6 mmol/L in diabetic ketoacidosis occasionally occur in patients with type 1 diabetes mellitus. The pattern of dyslipidemia is usually Fredrickson classification type lV. But it also exists in type III and type V. However, extreme triglyceridemia, triglyceride level exceeds 22.6 mmol/L, occur rarely in the modern era of insulin therapy. And the pattern is usually Fredrickson type I. The severe hypertriglyceridemia in diabetic ketoacidosis is mainly due to lipoprotein lipase deficiency, and secondly to insulin deficiency. The severity usually improves with insulin replacement. In patients with extreme hypertriglyceri-demia, serum electrolyte values of the patients are fallaciously low, and it leads to the misinterpretation of biochemical results and to the inappropriate treatment. We reported a case of a 25 years old female patient with diabetic ketoacidosis and extreme hypertriglyceridemia. At admission, the color of her serum was milky, her plasma triglyceride concentration was 144.7 mmol/L (12864 mg/dL), cholesterol was 25.5 mmol/L (982 mg/dl), and HDL-cholesterol was 0.77 mmol/L (40 mg/dL). The biochemical values at admission could not be measured. Empirical therapy was administered with the use of insulin and fluid. After the initial treatment with insulin and fluid, plasma triglyceride declined rapidly and was nearly normal after 72 hours. We also measured fasting blood glucose concentration and lipid profiles from her father and two sisters. Their plasma glucose and lipid profiles were normal.

 KDA
KDA
 First
First Prev
Prev





